Human Subjects Committee
The faculty, staff, and students of SUNY Oswego are obligated to comply with US Department of Health and Human Services regulations for the protection of human participants in research. Any member of the campus community participating in a research project (including but not limited to laboratory experiments, field studies, and interviews) is entitled to be free from possible injury and/or psychological harm and to maintain his/her personal privacy. The committee was formed to ensure that these guidelines are followed, therefore protecting human research participants studied by researchers on our campus.
The Human Subjects Committee (also known as SUNY Oswego's IRB) is comprised of members from diverse backgrounds to promote complete and adequate review of research activities covered by this assurance.
Before submitting a research protocol for review, please read through the
SUNY Oswego HSC Policies and Procedures Manual.
All new IRB protocols must be submitted through Google Forms. Before submitting you will be asked to log into your SUNY Oswego Google account if you have not done so already. Exempt and expedited reviews will take 1-2 weeks for review. Full committee reviews will occur at least monthly. If you think that your protocol is greater than minimal risk, or if it involves a protected class, please submit your protocol at least two (2) weeks in advance of the next scheduled HSC meeting.
- SUNY Oswego Human Subject Committee Research Protocol Form
- Microsoft Word version
- Google Docs version
- Informed Consent (Sample Documents)
- New General Informed Consent Form
- Minor Assent Form
- Minor Parent/Legal Guardian Consent Form
- Audio/Visual Recording Consent Form
- Submit your complete protocol and all associated documents
- Additional Investigators Addendum
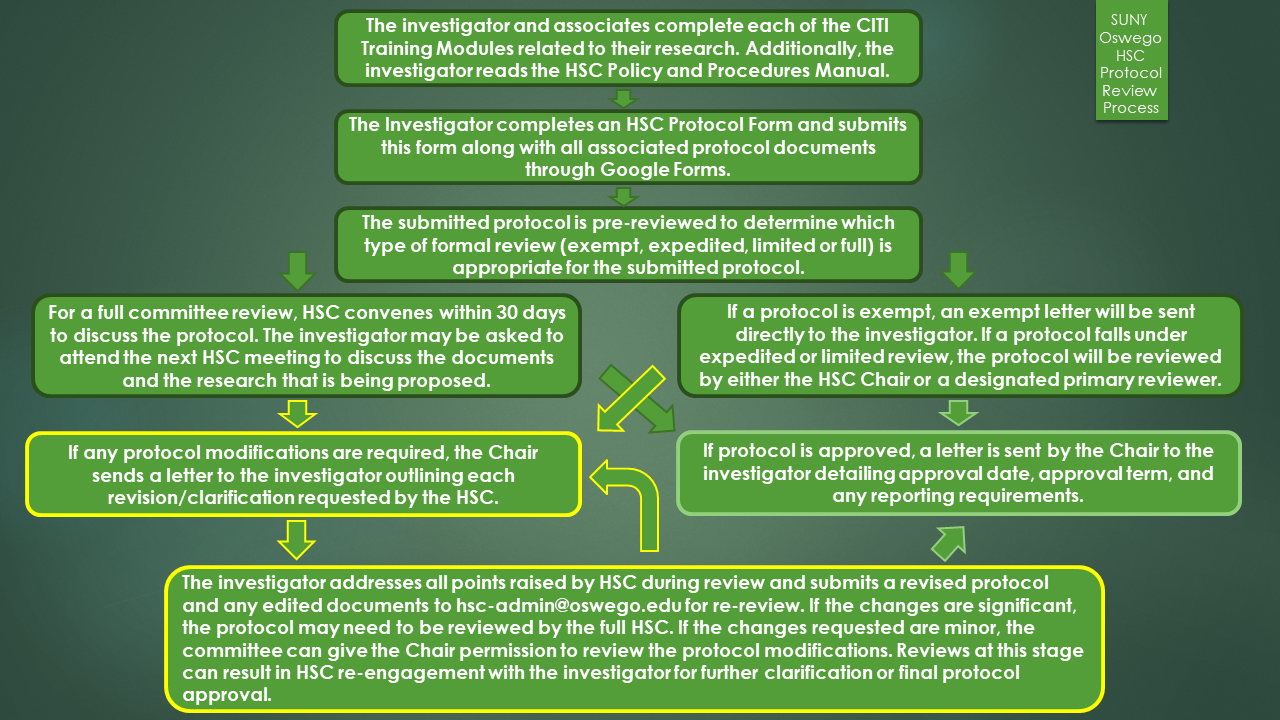
To satisfy Federal requirements, every study approved by the Human Subjects Committee (HSC) at SUNY Oswego is approved for at most one year.
Beginning Fall 2018, it is imperative that all individuals who will be conducting research involving human subjects complete the appropriate CITI Training modules.
To access training modules and to set up an account, please create a CITI Account and follow the prompts provided.
For assistance with this process, please review the guidance documentation below that is most applicable to your work:
45 CFR 46 - The Code of Federal Regulations that must be followed when conducting research with human subjects
Human Subject Regulations Decision Charts
If you have a course where students will he completing classroom projects that involve human participants, please carefully review the "SUNY Oswego HSC - Policy on Student Classroom Projects".
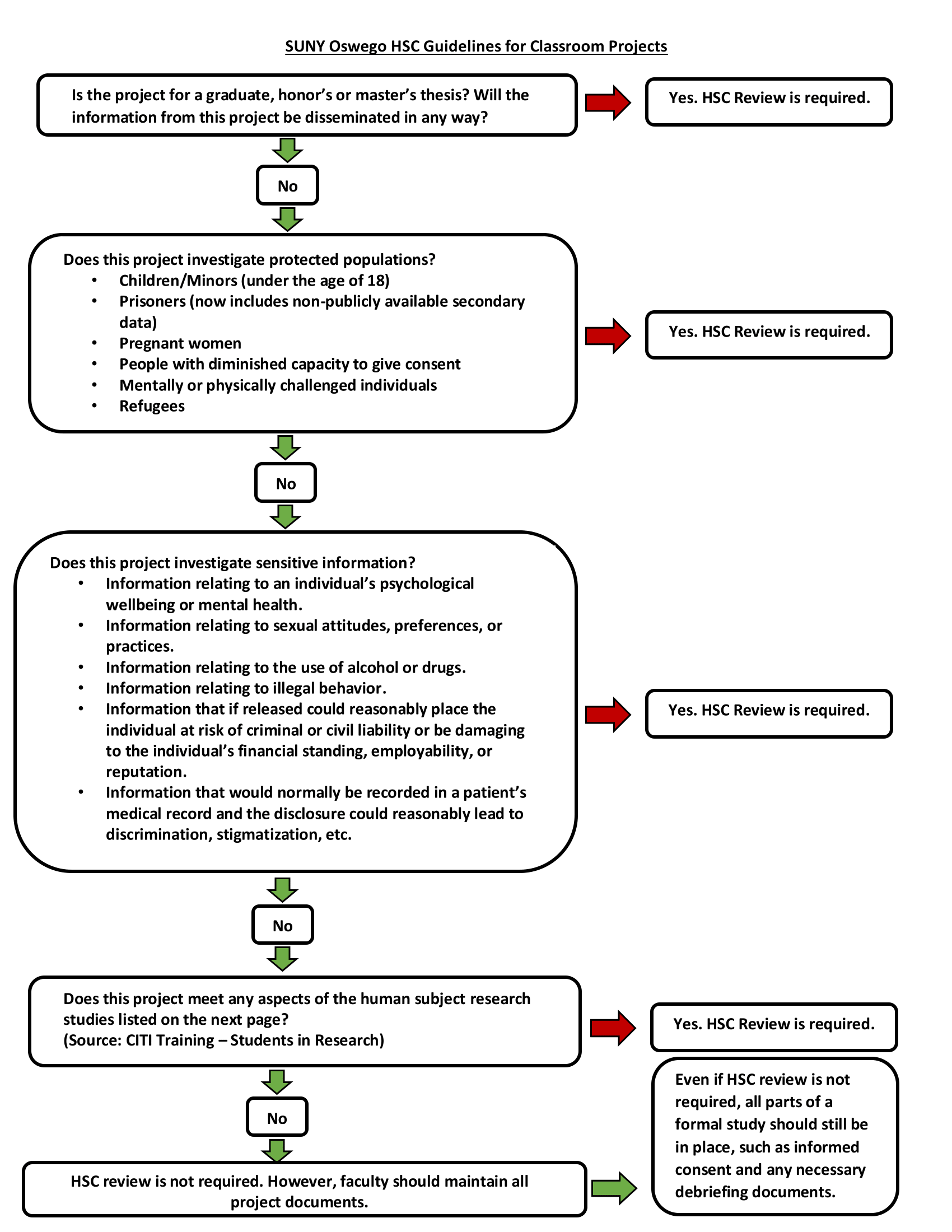
Please complete this Google Form to determine if your research may be exempt under the common rule. Be sure to submit the form when you have completed it. Your email and any information submitted will only be visible to HSC administrators. If you have completed this form and the CITI training, and you are still unsure about your research, please contact hsc-admin@oswego.edu.
Even if you believe that your research is exempt, you must still complete and submit a protocol application form. The HSC chair will determine if the research is exempt during the pre-review stage, and a letter of exempt status will be sent to your email for your records.
All active and ongoing research involving human subjects that is expedited or greater than minimal risk requires continual oversight by an IRB.
Therefore, if you are leaving SUNY Oswego, there are a few options available to you:
Transfer your studies to another institution– The Oswego HSC will work with your new institution to ensure that there is continued coverage for your research study. As soon as possible, please let ORSP know that you are leaving Oswego and that you wish to have your study transferred to your new institution.
Transfer to another PI– If taking your study with you is not an option, the study may remain open if another PI is identified. Once the new PI is ready, you will submit a modification to change the PI on your study.
Close the study– If you are not interested in continuing with your study or are just not able to carry on for various reasons, you will need to close your study. If your study is funded, it is always a good idea to check in with your funding agency about whether closing your study is possible. You will also want to think about what you will do with the data that has been collected. And a friendly reminder – if the data are identifiable, it is not permissible to analyze these data without current and active IRB approval.
Please contact hsc-admin@oswego.edu if you have any further questions, or to begin this process.
Individual Investigator Agreement (IIA)
This is a formal written agreement between an institution conducting research and an individual investigator who is collaborating on the research project, by which the institution agrees to extend its Federal-Wide Assurance (FWA) about human subjects research to the individual, and by which the independent investigator agrees to fulfill specified expectations and responsibilities. For example, this agreement could apply to a former student working after graduation with their faculty mentor, collaborating professional in the community with specific expertise, or community partners that do not have an FWA. The IIA is signed by the
- Individual Investigator
- SUNY Oswego Principal Investigator (PI)
- SUNY Oswego Institutional Official or designee
Deborah Furlong, Institutional Research and Assessment
Sabine Ingerson, Community Member
Elizabeth Keida, Health Promotion and Wellness
Sungeun Kim, Electrical and Computer Engineering
Bastian Tenbergen, Computer Science
Michele Thornton, School of Business
If your protocol is greater than minimal risk, or if it involves a protected class, HSC will meet to review and return a decision. Please allow enough time for the committee to review before the anticipated start date of work.
Fall 2023 Meeting Dates are TBD.If your protocol is greater than minimal risk, or if it involves a protected class, HSC will meet to review and return a decision. Please allow enough time for the committee to review before the anticipated start date of work.
Spring 2024 Meeting Dates are TBD.
If you have any questions about or related to HSC, or the process of submitting a protocol, please contact:
Information for Members
The following links are accessible to HSC members only when signed into their oswego.edu email account.
- Meeting Minutes
- Meeting Agendas (Upcoming and past agendas)
- Documents Under Discussion
- Protocols Under Discussion
- Active Protocols


